 The story of sickle-cell anemia and its malaria-protective effects is a textbook case how environmental context determines the fitness of a given genetic profile. However, the evolution of human blood disorders in response to selection from malaria parasites might be more complicated than that textbook story.
The story of sickle-cell anemia and its malaria-protective effects is a textbook case how environmental context determines the fitness of a given genetic profile. However, the evolution of human blood disorders in response to selection from malaria parasites might be more complicated than that textbook story.

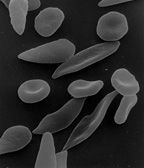 Malaria-causing parasites (dark-stained) among human red blood cells (top), and “sickled” red blood cells (bottom). Photos via WikiMedia Commons.
Malaria-causing parasites (dark-stained) among human red blood cells (top), and “sickled” red blood cells (bottom). Photos via WikiMedia Commons.Malaria is caused by mosquito-spread parasites that attack their hosts’ oxygen-bearing red blood cells. A particular mutation in the gene that codes for part of the hemoglobin molecule – the molecule that actually stores oxygen inside red blood cells – leads to deformed, sickle-shaped, blood cells. People who carry two copies of the sickle cell gene develop sickle-cell disease, in which the sickle-shaped cells reduce oxygen transport efficiency and interfere with blood circulation. People with only one copy of the sickle-cell gene are healthy, and better able to resist malaria infection than those with no copies. The textbook story is that, in regions where malaria is common, such as sub-Saharan Africa, the advantage of malaria resistance is enough to offset the fitness risk of carrying the sickle-cell gene – that one-fourth of children born to parents who each have one copy of the gene will themselves have two copies and develop sickle-cell disease.
However, there are regions like the Mediterranean where malaria has historically been prevalent, but in which the human population hasn’t evolved the higher frequency of sickle-cell genes that you’d expect from the scenario outlined above. A new paper in PNAS demonstrates that this may be because of interactions between the sickle-cell gene and another genetic blood disorder, thalassemia [$a].
Thalassemia is a class of genetic disorders affecting the protein subunits that comprise hemoglobin. Each hemoglobin molecule is formed by binding together two “alpha”-type subunits, and two “beta”-type subunits. If there is a shortage of correctly-formed subunits of either type, then hemoglobin formation is impaired, resulting in anemia or (if the mutation stops subunit production altogether) death. However, like sickle-cell genes, thalassemic mutations can confer resistance to malaria; and if alpha-thalassemia is paired with beta-thalassemia, the reduced production of both subunits can balance out.
As it happens, in combination with alpha-thalassemia, the sickle-cell gene’s malaria protection is neutralized. Using population genetic models, the new study’s authors show that this effect may have actively prevented the sickle-cell gene from establishing in the Mediterranean, where alpha- and beta-thalassemias are more common than in Africa. In the Mediterranean, the presence of beta-thalassemia genes reduces the fitness cost of (mild) alpha-thalassemia genes; and in the presence of alpha-thalassemia genes, the sickle-cell gene confers no protection to people with one copy but still induces sickle-cell disease in people with two copies.
These interactions between genes are called epistasis, and they can have dramatic impacts on evolution. Although I haven’t seen many cases as well-characterized as this one, epistasis is probably widespread in the complex systems of genomes, where thousands of regulatory and protein-coding genes interact to build living things.
References
Penman, B., Pybus, O., Weatherall, D., & Gupta, S. (2009). Epistatic interactions between genetic disorders of hemoglobin can explain why the sickle-cell gene is uncommon in the Mediterranean Proc. Nat. Acad. Sci. USA, 106 (50), 21242-6 DOI: 10.1073/pnas.0910840106
 Saiga antelope. Photo via Nothing in Biology Makes Sense.
Saiga antelope. Photo via Nothing in Biology Makes Sense.

